'HumaPen' insulin injector
Object No. 2013/24/1
Humulin was the world's first approved genetically engineered human therapeutic. It is insulin that is made by bacteria but chemically and physically identical to insulin produced by a human pancreas. Human insulin is used daily by millions of people who live with diabetes and are unable to make their own insulin. It replaces therapeutic insulin derived from cattle and pigs, both of which have adverse side effects in some people. Insulin has been injected subcutaneously to treat diabetes since 1938. Insulin pens are more convenient to transport and use than glass vials and syringes which were the only injecting technology available until 1985. They provide repeatedly accurate doses and are discreet to use. Insulin is a naturally-occurring hormone that is secreted by the pancreas of a healthy person. It controls the amount of glucose in the blood by allowing cells to take glucose from the blood and use it for energy. People who cannot produce insulin have uncontrolled blood sugar which can damage blood vessels, eyes, nerves and kidneys. Sandra McEwen, Principal Curator, Biosciences and Built Environment, 2013
Loading...
Summary
Object Statement
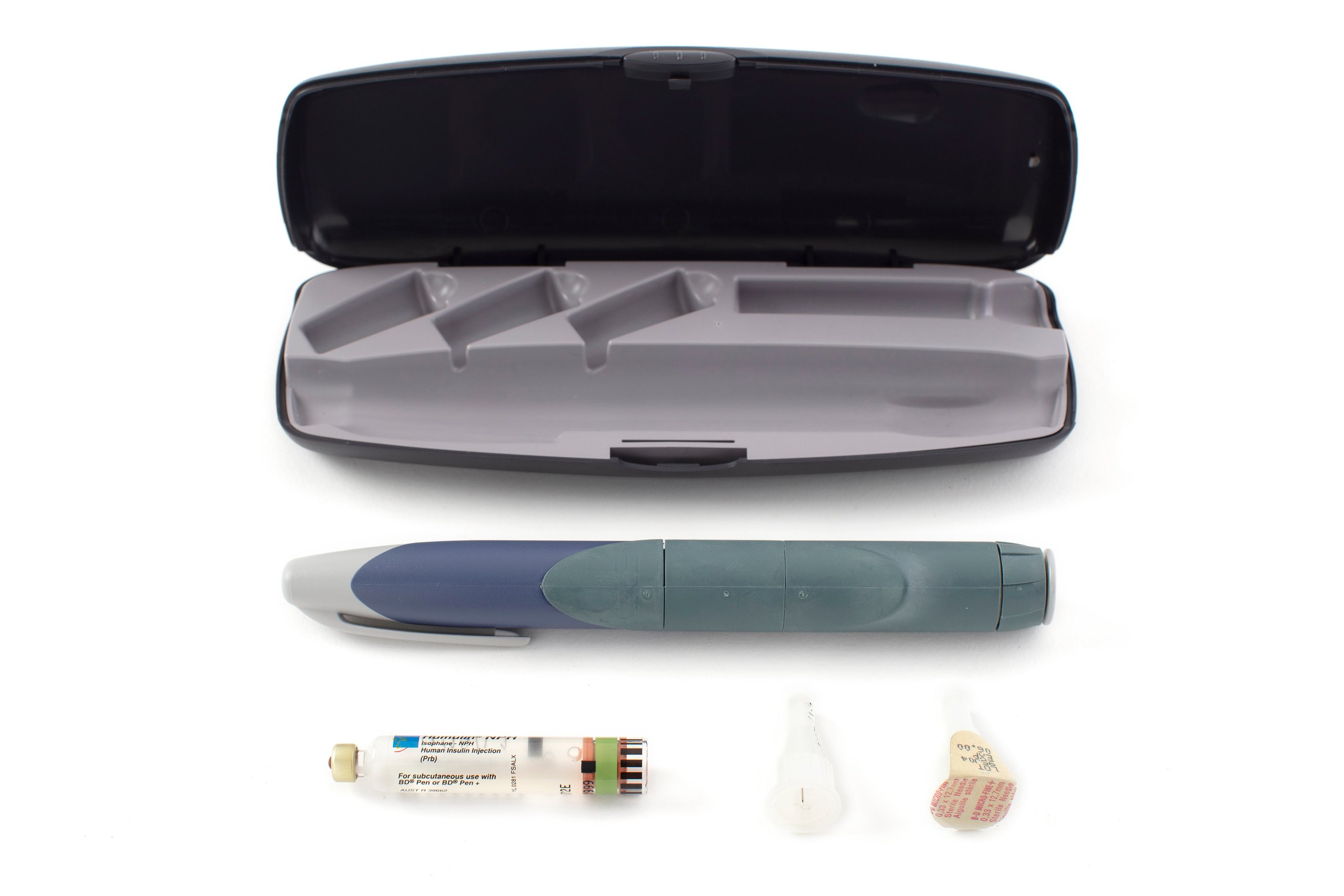
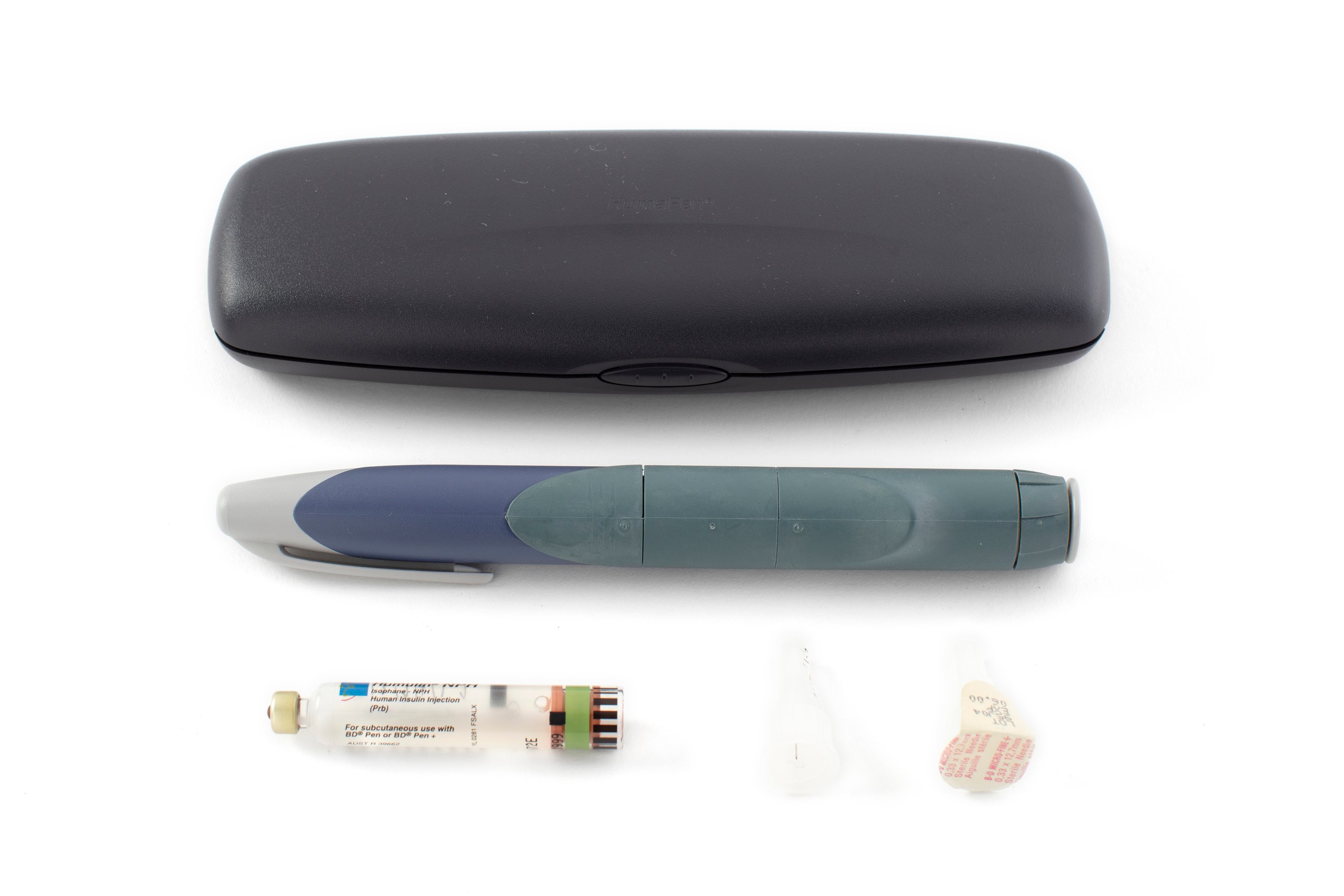
Insulin injector, 'HumaPen', and accessories including vial, needle tips (2) and case, made using recombinant DNA, plastic / glass / metal / rubber, Eli Lilly and Company, Australia, 1997-1999
Physical Description
HumaPen insulin injector with a cap on one end and a variable dose regulator at the other. The injector contains a 1.5mL glass cartridge of Humulin R human insulin. The injector is accompanied by a second 3mL glass cartridge of Humulin NPH human insulin and two needle tips with protective plastic covers. The components are held within a small purpose-made plastic case with a push lock.
DIMENSIONS
Height
70 mm
Width
187 mm
Depth
85 mm
PRODUCTION
Notes
This HumaPen was made by Eli Lilly and Company in 1997 or 1998. The cartridges of Humulin were made by Eli Lilly and Company in 1998. Humulin is made using recombinant DNA technology. This involves changing the genetic material of a laboratory strain of Escherichia coli bacteria to include a segment of DNA that codes for human insulin. The bacteria act like little factories, reading the synthetic DNA and assembling the insulin. The insulin is then isolated from the bacteria, purified and packaged. Regular insulin (Humulin R) has a rapid onset of action but is short acting. It begins to reduce blood sugar 30 minutes after injection and lasts for 6 to 8 hours. It has a peak effect about 1 to 3 hours after injection. NPH insulin has an intermediate duration of action. It starts acting about 2 hours following injection and lasts about 18 to 26 hours. It has a peak effect about 4 to 12 hours after injection.
HISTORY
Notes
The HumaPen was first released for use in Australia in 1997. Humulin R and Humulin NPH were first distributed for use in Australia in glass vials in 1986. They were released in Australia in glass cartridges in 1992. In 1978, in Herbert Boyer's laboratory at the University of California, scientists constructed a synthetic version of the genetic code for human insulin and inserted it into a bacterium, Escherichia coli. The bacteria incorporated the new genetic code into it's plasmid, a loop of DNA carrying its own genetic material. The bacteria then proceeded to make human insulin that was indistinguishable from insulin produced by a human pancreas. This was a turning point in the development of modern biotechnology. Herbert Boyer was a founder of Genentech, the Californian company that developed Humulin. Humulin is the brand name of a group of biosynthetic human insulin products that were approved by the U.S. Food and Drug Administration (FDA) in 1982. Genentech's commercial partner in the project was Eli Lilly and Company. Humulin was licensed to and manufactured by Eli Lilly. Injection pens were first developed by Novo Nordisk in 1985.
SOURCE
Credit Line
Acquired 2013
Acquisition Date
26 February 2013

Copyright for the above image is held by the Powerhouse and may be subject to third-party copyright restrictions. Please submit an Image Licensing Enquiry for information regarding reproduction, copyright and fees. Text is released under Attribution-Non Commercial-No Derivative licence.
Image Licensing Enquiry
Object Enquiry